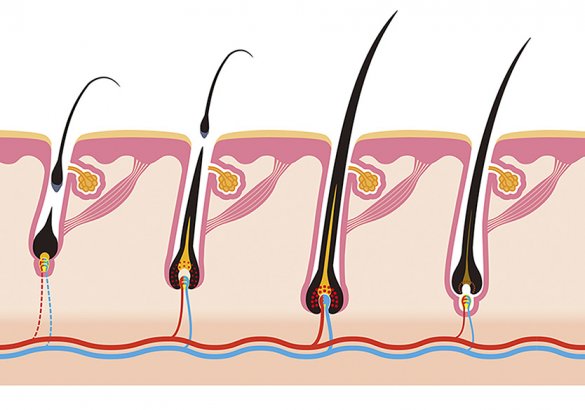
The male and female subjects taking part in a clinical study on Trinov hair-loss lotion have confirmed that daily application of the product for 6 months leads to a significant reduction in hair loss and confirmed that hair becomes stronger, healthier and fuller-bodied. The same subjects also reported that the lotion is readily absorbed and does not alter the condition of the scalp, whilst giving it a pleasant scent.
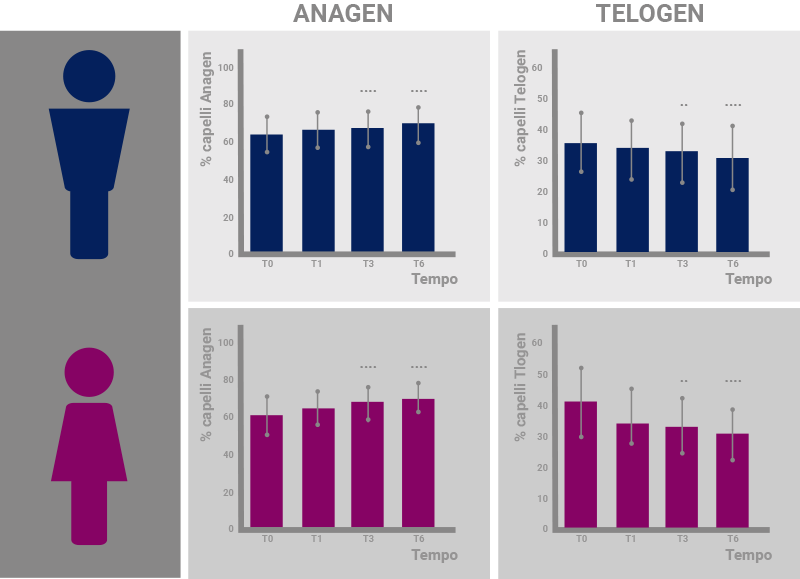
Results of the study regarding the percentage of hairs in anagen phase (left) and telogen phase (right) in men (above, blue) and women (below, pink). The improvement is significant from T3 in men and from T1 in women.*
*”A liposome-based formulation containing equol, dihomo-γ-linolenic acid and propionyl-L-carnitine to prevent and treat hair loss: A prospective investigation” G.Brotzu, A.M.Fadda, M.L.Manca, T.Manca, F.Marongiu, M.Campisi, F.Consolaro; Dermatologic Therapy, Ott.2018
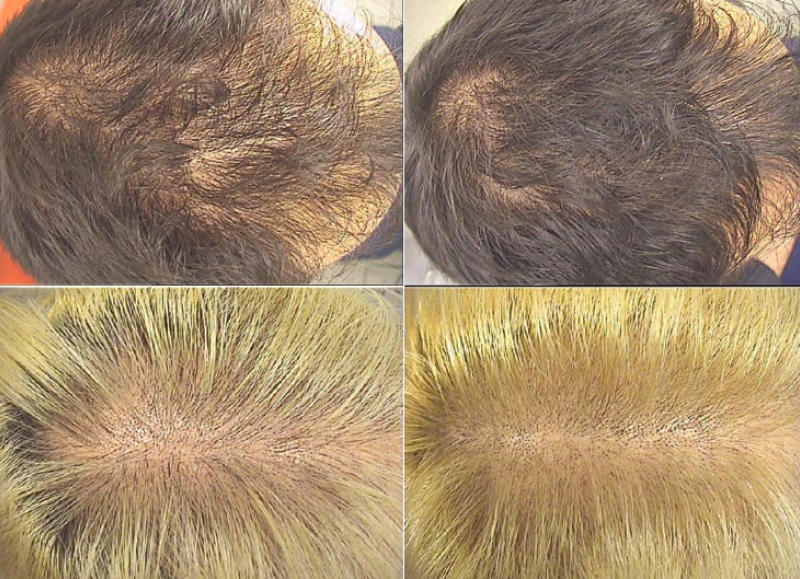
Two representative cases of one male volunteer and one female volunteer who used the lotion for 6 months (a, c: before use of the treatment; b, d: after 6 months’ use of the treatment).*
*”A liposome-based formulation containing equol, dihomo-γ-linolenic acid and propionyl-L-carnitine to prevent and treat hair loss: A prospective investigation” G.Brotzu, A.M.Fadda, M.L.Manca, T.Manca, F.Marongiu, M.Campisi, F.Consolaro; Dermatologic Therapy, Ott.2018
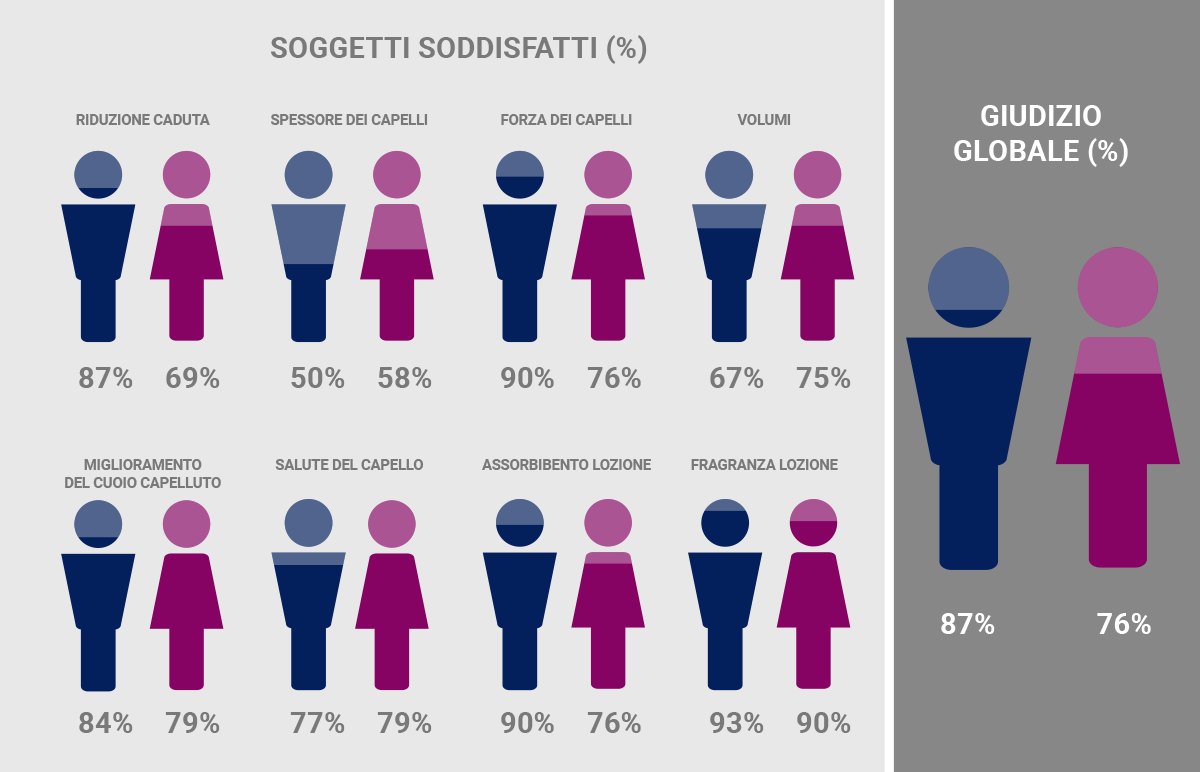
Percentage of male volunteers (blue) and female volunteers (pink) who said that they were very or quite satisfied with the characteristics and efficacy of the lotions used. Most subjects (≥ 50%) expressed good satisfaction.*
*”A liposome-based formulation containing equol, dihomo-γ-linolenic acid and propionyl-L-carnitine to prevent and treat hair loss: A prospective investigation” G.Brotzu, A.M.Fadda, M.L.Manca, T.Manca, F.Marongiu, M.Campisi, F.Consolaro; Dermatologic Therapy, Ott.2018
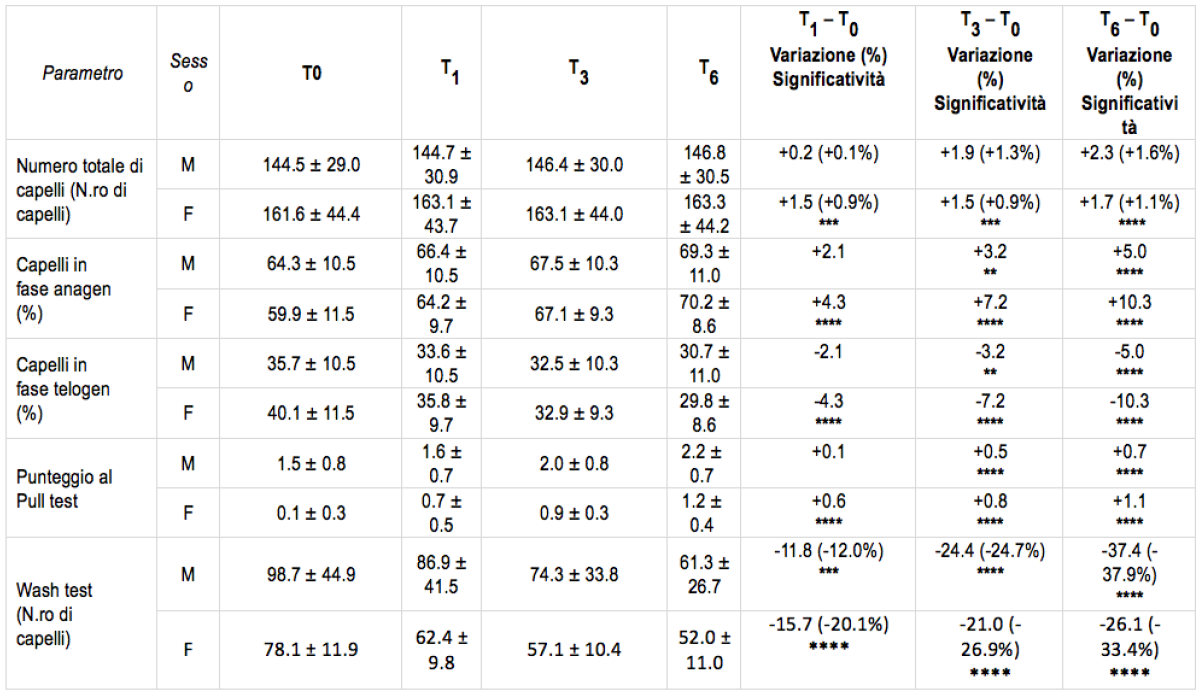
Table 1. Results of the study. The asterisks indicate the significance of the statistical tests compared to the baseline condition, before treatment. *, p p<0.05; **, p p<0.01; ***, p p<0.001; ****, p p<0.0001*
*”A liposome-based formulation containing equol, dihomo-γ-linolenic acid and propionyl-L-carnitine to prevent and treat hair loss: A prospective investigation” G.Brotzu, A.M.Fadda, M.L.Manca, T.Manca, F.Marongiu, M.Campisi, F.Consolaro; Dermatologic Therapy, Ott.2018